CD45 antibody | YKIX716.13








Rat anti Dog CD45
- Product Type
- Monoclonal Antibody
- Clone
- YKIX716.13
- Isotype
- IgG2b
- Specificity
- CD45
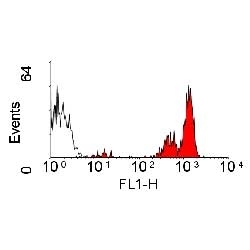
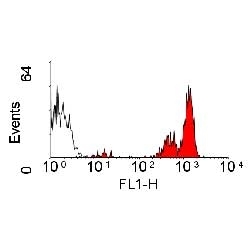
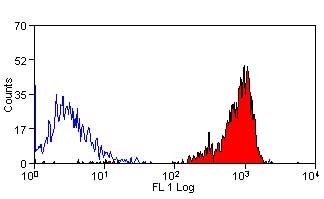
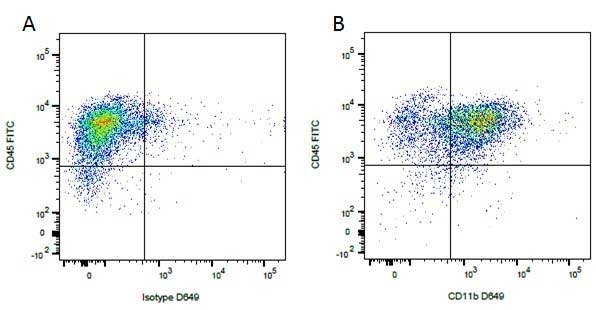
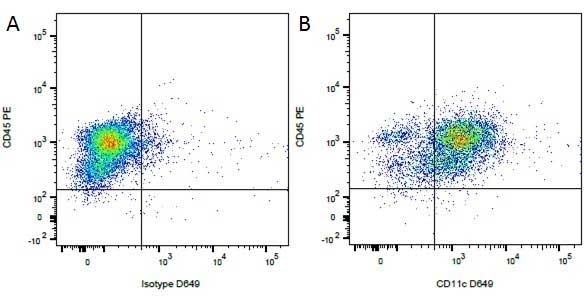
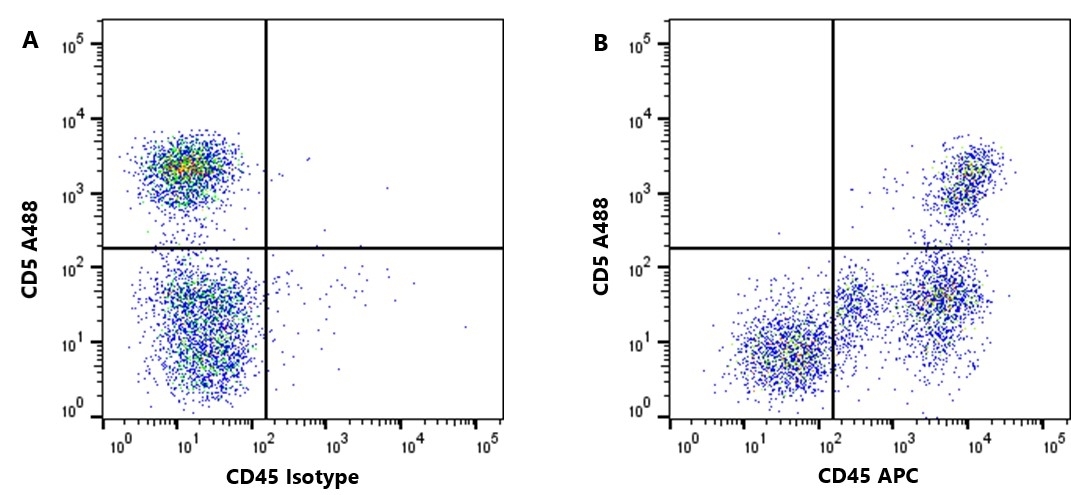
| Rat anti Dog CD45 antibody, clone YKIX716.13 recognizes canine CD45 also known as leukocyte common antigen lustered as Canine CD45 in the First Canine Leukocyte Antigen Workshop (CLAW). Clone YKIX 716.13: immunoprecipitates an antigen of ~180/200 kDa from Con-A blasts (Cobbold et al. 1994). CD45 is expressed on all leukocytes in canine peripheral blood. Rat anti Dog CD45 antibody, clone YKIX716.13 reacts with CD45 on all outbred mongrels and beagles tested and may be against CD45RB isoform. |
- Target Species
- Dog
- Product Form
- Purified IgG - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein G from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
- 0.09% sodium azide (NaN3)
- Carrier Free
- Yes
- Immunogen
- Canine thymocytes.
- Approx. Protein Concentrations
- IgG concentration 1.0 mg/ml
- Fusion Partners
- Spleen cells from immunized DA rats were fused with cells of the Y3/Ag1.2.3 rat myeloma cell line.
- Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | 1/50 | 1/100 | |
| Immunoprecipitation |
| Description | Product Code | Applications | Pack Size | List Price | Your Price | Quantity | |
|---|---|---|---|---|---|---|---|
| Rat IgG2b Negative Control | MCA6006GA | F | 0.1 mg |
|
Log in | ||
| List Price | Your Price | ||||||
|
|
Log in | ||||||
| Description | Rat IgG2b Negative Control | ||||||
References for CD45 antibody
-
Cobbold, S. & Metcalfe, S. (1994) Monoclonal antibodies that define canine homologues of human CD antigens: summary of the First International Canine Leukocyte Antigen Workshop (CLAW).
Tissue Antigens. 43 (3): 137-54. -
Zentek, J. et al. (2002) Morphology and immunopathology of the small and large intestine in dogs with nonspecific dietary sensitivity.
J Nutr. 132: 1652S-4S. -
Sanchez, M.A. et al. (2004) Organ-specific immunity in canine visceral leishmaniasis: analysis of symptomatic and asymptomatic dogs naturally infected with Leishmania chagasi.
Am J Trop Med Hyg. 70: 618-24. -
Reis, A.B. et al (2006) Phenotypic features of circulating leucocytes as immunological markers for clinical status and bone marrow parasite density in dogs naturally infected by Leishmania chagasi.
Clin Exp Immunol.146: 303-11. -
Comazzi, S. et al. (2006) Flow cytometric patterns in blood from dogs with non-neoplastic and neoplastic hematologic diseases using double labeling for CD18 and CD45.
Vet Clin Pathol. 35: 47-54. -
Stein, V.M. et al. (2008) Immunophenotypical characterization of monocytes in canine distemper virus infection.
Vet Microbiol. 131:237-46. -
Tominaga, M. et al. (2010) Flow cytometric analysis of peripheral blood and tumor-infiltrating regulatory T cells in dogs with oral malignant melanoma.
J Vet Diagn Invest. 22: 438-41. -
Hunter, M.J. et al. (2011) Gene therapy of canine leukocyte adhesion deficiency using lentiviral vectors with human CD11b and CD18 promoters driving canine CD18 expression.
Mol Ther. 19: 113-21.
View The Latest Product References
-
Giantin, M. et al. (2013) Evaluation of tyrosine-kinase receptor c-KIT (c-KIT) mutations, mRNA and protein expression in canine leukemia: might c-KIT represent a therapeutic target?
Vet Immunol Immunopathol. 152: 325-32. -
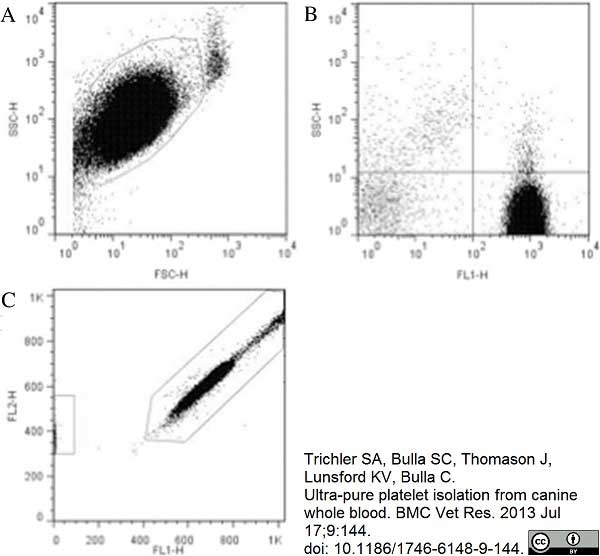
Trichler, S.A. et al. (2013) Ultra-pure platelet isolation from canine whole blood.
BMC Vet Res. 9: 144. -
Aricò, A. et al. (2013) The role of vascular endothelial growth factor and matrix metalloproteinases in canine lymphoma: in vivo and in vitro study.
BMC Vet Res. 9: 94. -
Michael, H.T. et al. (2013) Isolation and characterization of canine natural killer cells.
Vet Immunol Immunopathol. 155 (3): 211-7. -
Aresu, L. et al. (2014) VEGF and MMP-9: biomarkers for canine lymphoma.
Vet Comp Oncol. 12: 29-36. -
Gelain, M.E. et al. (2014) CD44 in canine leukemia: analysis of mRNA and protein expression in peripheral blood.
Vet Immunol Immunopathol. 159 (1-2): 91-6. -
Zeira, O. et al. (2015) Adult autologous mesenchymal stem cells for the treatment of suspected non-infectious inflammatory diseases of the canine central nervous system: safety, feasibility and preliminary clinical findings.
J Neuroinflammation. 12: 181. -
Salinas, L.T. et al. (2015) Mesenchymal stem cells do not exert direct beneficial effects on CNS remyelination in the absence of the peripheral immune system.
Brain Behav Immun. 50: 155-65. -
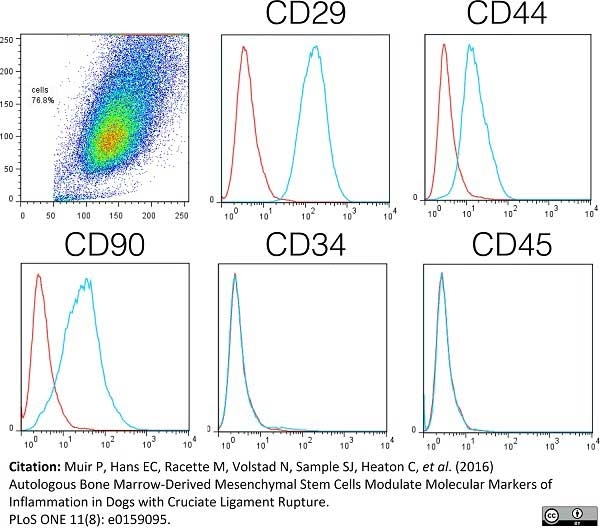
Muir, P. et al. (2016) Autologous Bone Marrow-Derived Mesenchymal Stem Cells Modulate Molecular Markers of Inflammation in Dogs with Cruciate Ligament Rupture.
PLoS One. 11 (8): e0159095. -
Bonnefont-Rebeix, C. et al. (2016) Characterization of a novel canine T-cell line established from a spontaneously occurring aggressive T-cell lymphoma with large granular cell morphology.
Immunobiology. 221 (1): 12-22. -
Poggi, A. et al. (2017) Prognostic significance of Ki67 evaluated by flow cytometry in dogs with high-grade B-cell lymphoma.
Vet Comp Oncol. 15 (2): 431-40. -
Nishimura, T. et al. (2017) Feeder-independent canine induced pluripotent stem cells maintained under serum-free conditions.
Mol Reprod Dev. 84 (4): 329-39. -
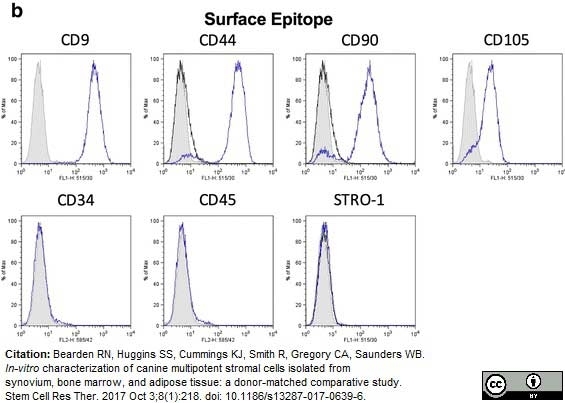
Bearden, R.N. et al. (2017) In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: a donor-matched comparative study.
Stem Cell Res Ther. 8 (1): 218. -
Hansmann, F. et al. (2018) Beneficial and detrimental impact of transplanted canine adipose-derived stem cells in a virus-induced demyelinating mouse model.
Vet Immunol Immunopathol. 202: 130-40. -
Reineking, W. et al. (2018) Canine primary jejunal and colonic epithelial cells predominantly express TLR5 and TLR9 but do not change TLR expression pattern after stimulation with certain Toll-like receptor ligands.
Vet Immunol Immunopathol. 206: 16-24. -
Martini, V. et al. (2019) Prognostic role of non-neoplastic lymphocytes in lymph node aspirates from dogs with diffuse large B-cell lymphoma treated with chemo-immunotherapy.
Res Vet Sci. 125: 130-5. -
Crain, S.K. et al. (2019) Extracellular Vesicles from Wharton's Jelly Mesenchymal Stem Cells Suppress CD4 Expressing T Cells Through Transforming Growth Factor Beta and Adenosine Signaling in a Canine Model.
Stem Cells Dev. 28 (3): 212-226. -
Wolf-Ringwall, A. et al. (2020) Prospective evaluation of flow cytometric characteristics, histopathologic diagnosis and clinical outcome in dogs with naïve B-cell lymphoma treated with a 19-week CHOP protocol.
Vet Comp Oncol. 18 (3): 342-52. -
Sayag, D. et al. (2020) Proof-of-concept study: Evaluation of plasma and urinary electrolytes as markers of response to L-asparaginase therapy in dogs with high-grade lymphoma.
Vet Clin Pathol. 49 (3): 476-83. -
Lee, J. et al. (2021) Canine Natural Killer Cell-Derived Exosomes Exhibit Antitumor Activity in a Mouse Model of Canine Mammary Tumor.
Biomed Res Int. 2021: 6690704. -
Wi, H. et al. (2021) Immunosuppression-enhancing effect of the administration of allogeneic canine adipose-derived mesenchymal stem cells (cA-MSCs) compared with autologous cA-MSCs in vitro.
J Vet Sci. 22 (5): e63. -
Grudzien, M. et al. (2021) A newly established canine NK-type cell line and its cytotoxic properties.
Vet Comp Oncol. 19 (3): 567-577. -
Stein, L. et al. (2021) Immunophenotypic Characterization of Canine Nodal T-Zone Lymphoma.
Vet Pathol. 58 (2): 288-92. -
Grandi, F. et al. (2022) Immunophenotypic and molecular profile of cancer stem-cell markers in ex vivo canine transmissible venereal tumour (CTVT).
Vet Med Sci. 8 (6): 2297-306. -
Martini, V. et al. (2018) A retrospective study of flow cytometric characterization of suspected extranodal lymphomas in dogs.
J Vet Diagn Invest. 30 (6): 830-6. -
Sheng, R. et al. (2023) Prognostic significance of CD25 expression in dogs with a noninvasive diagnosis of B-cell lymphoma treated with CHOP chemotherapy.
Vet Comp Oncol. 21 (1): 28-35. -
Kim, J.H. et al. (2024) Hemangiosarcoma Cells Promote Conserved Host-Derived Hematopoietic Expansion.
Cancer Res Commun. 16 May [Epub ahead of print]. -
Benavides, F.P. et al. (2021) Intrathecal Transplantation of Autologous and Allogeneic Bone Marrow-Derived Mesenchymal Stem Cells in Dogs.
Cell Transplant. 30: 9636897211034464.
MCA1042GA
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Anti-Dog ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up