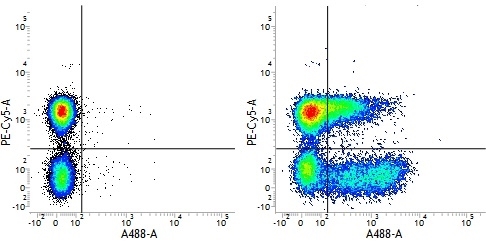
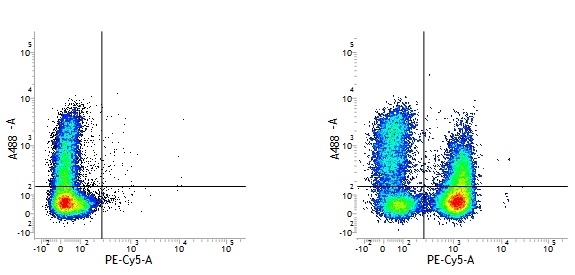
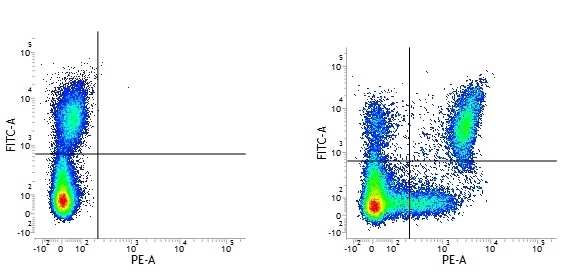
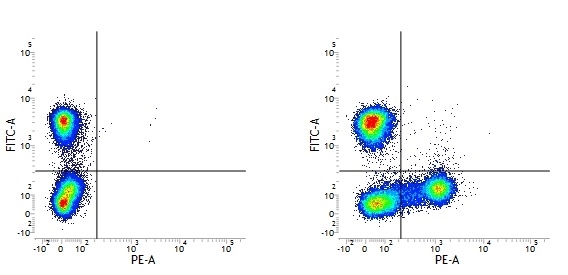
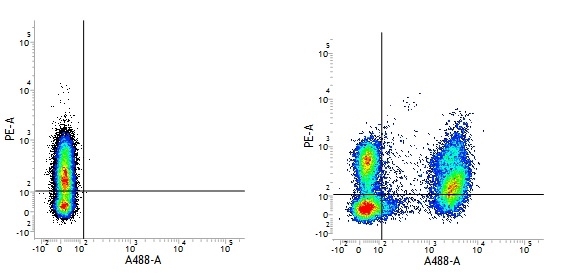
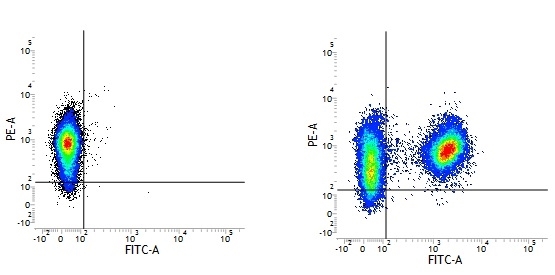
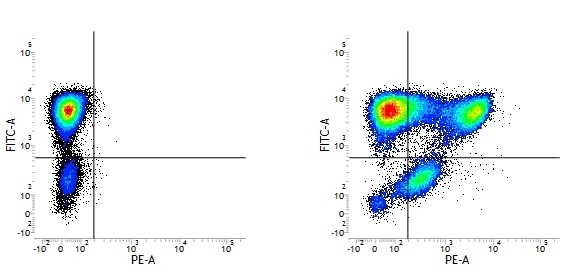
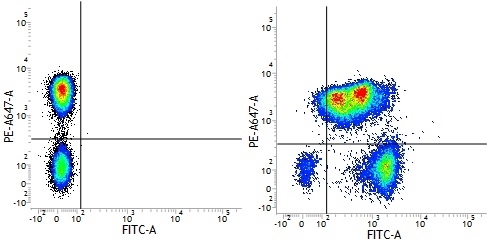
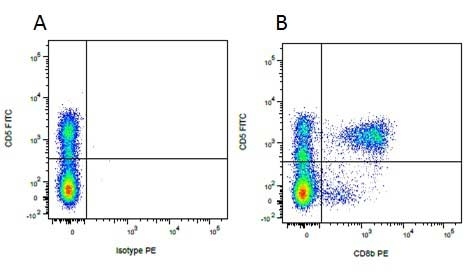
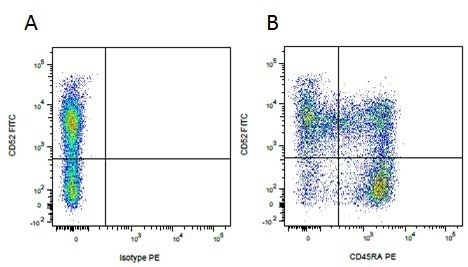
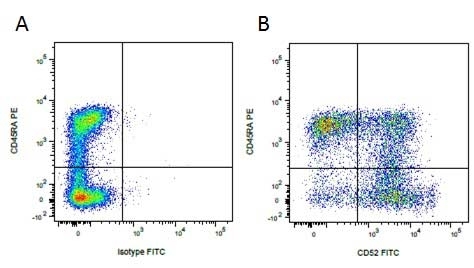
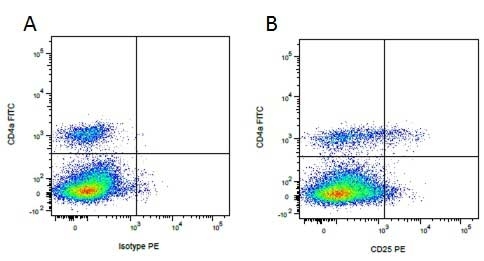
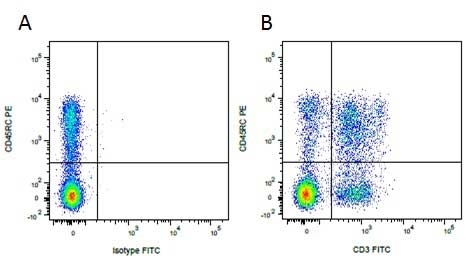
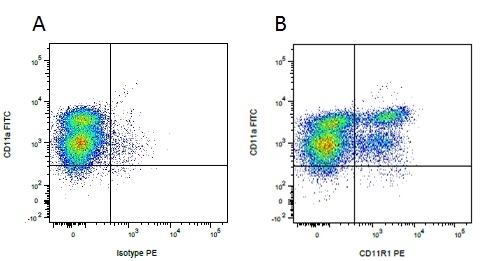
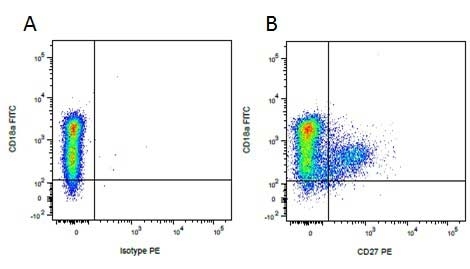
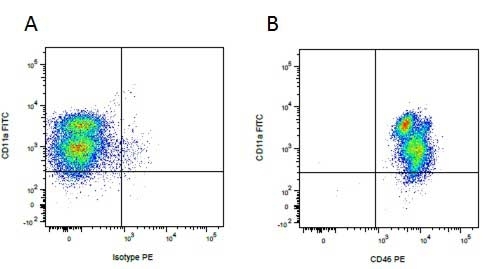
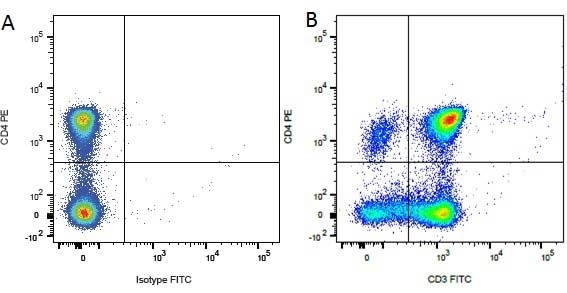
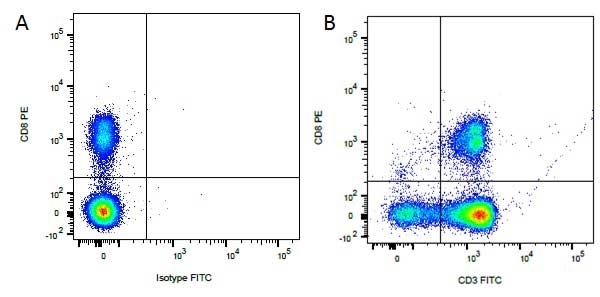
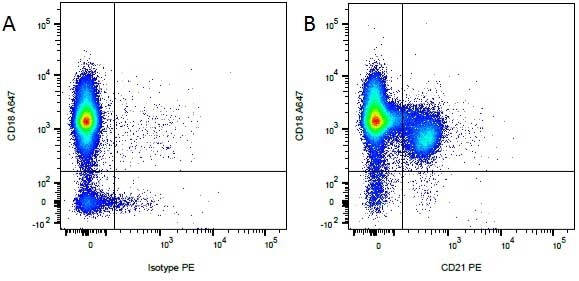
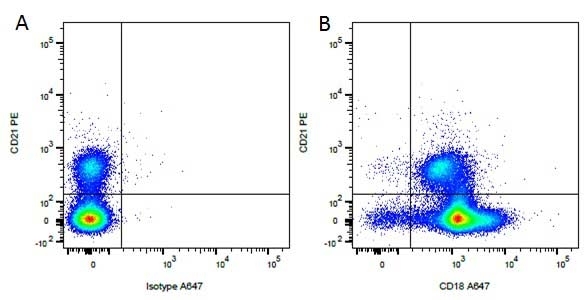
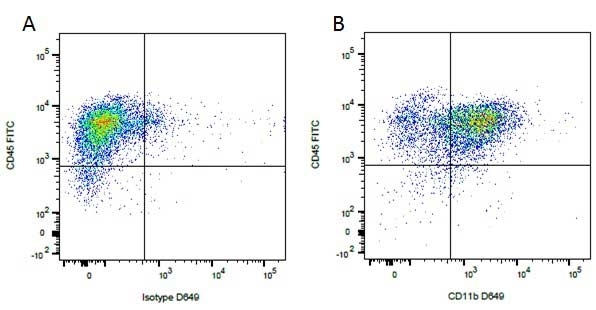
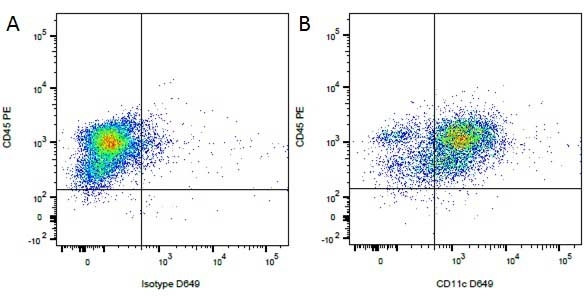
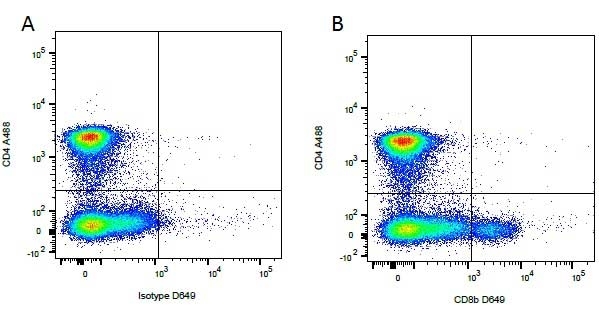
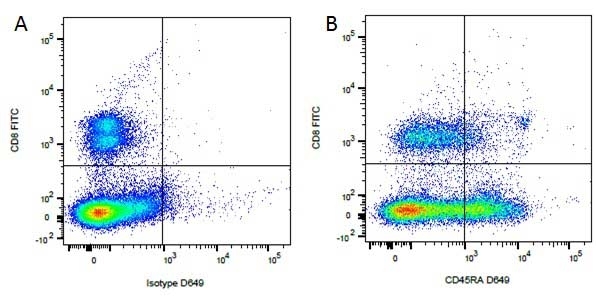
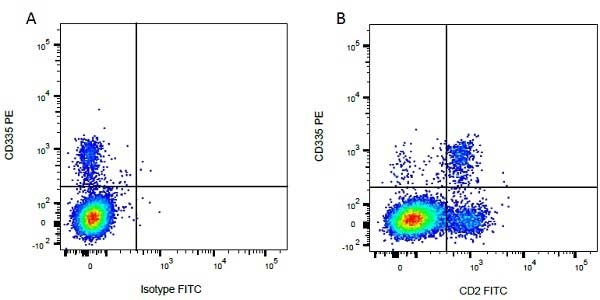
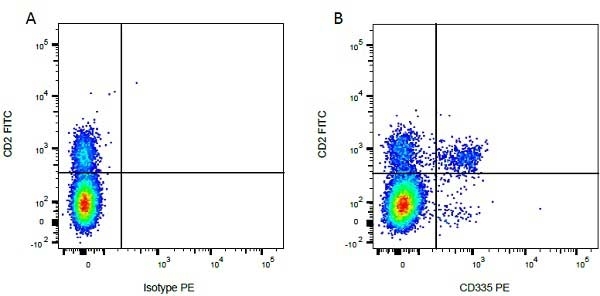
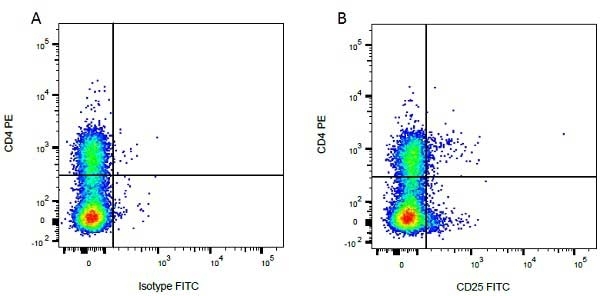
Mouse IgG1 Negative Control antibody

























































Mouse IgG1 Negative Control:Pacific Blue®
- Product Type
- Negative/Isotype Control
- Isotype
- IgG1
- Specificity
- Mouse IgG1 Negative Control
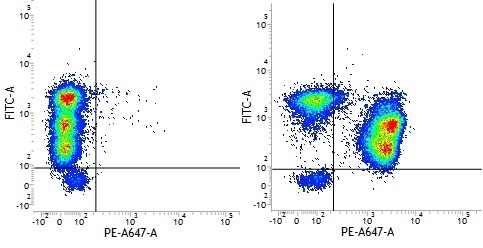
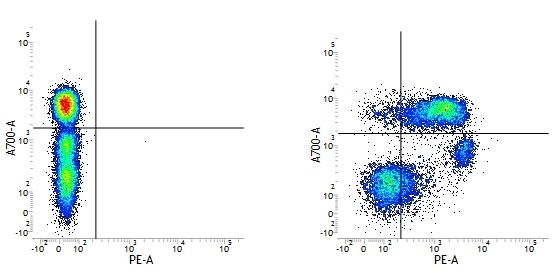
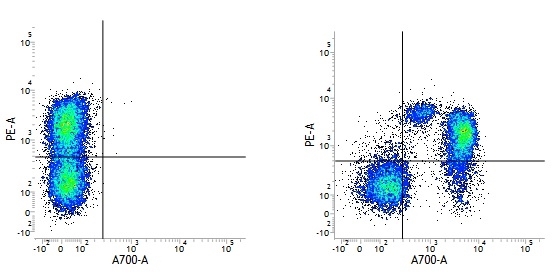
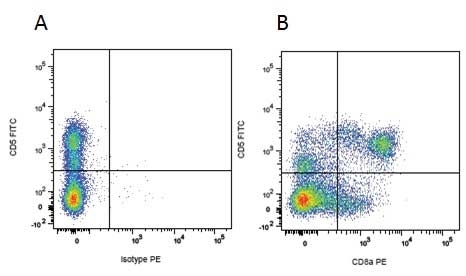
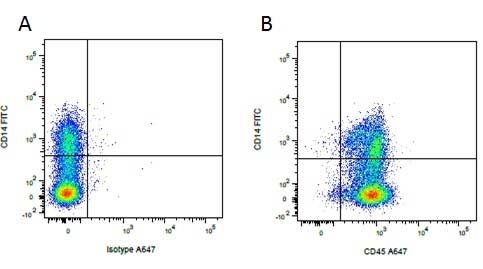
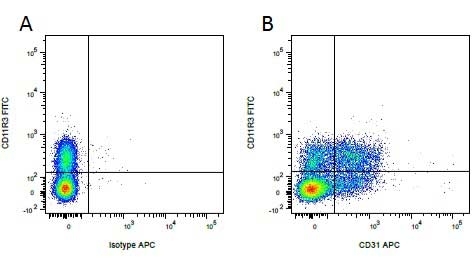
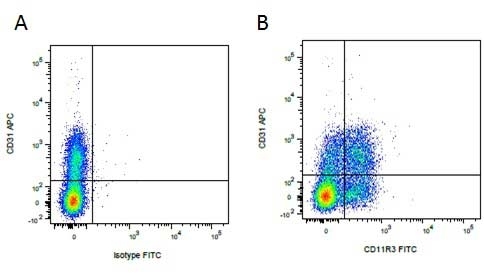
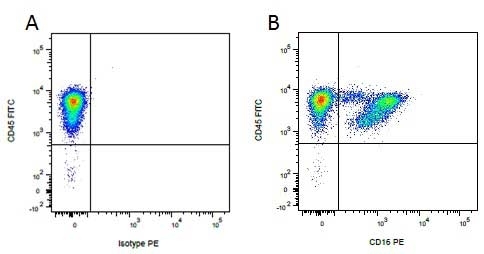
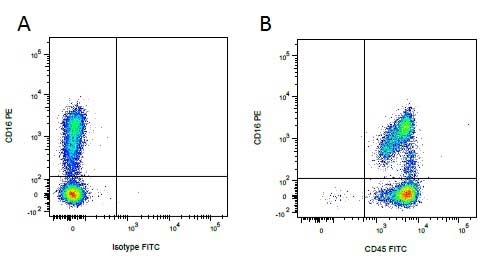
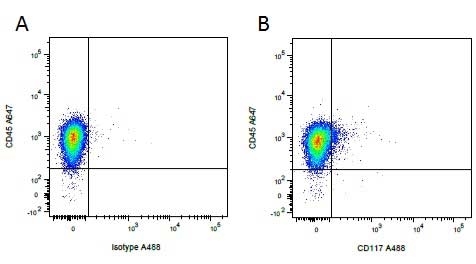
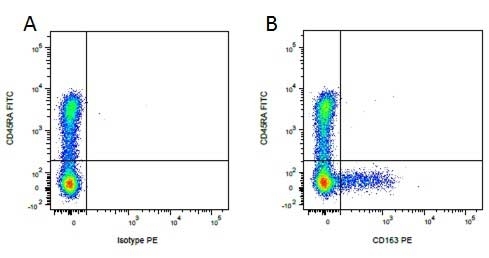
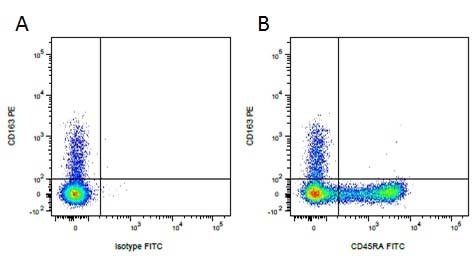
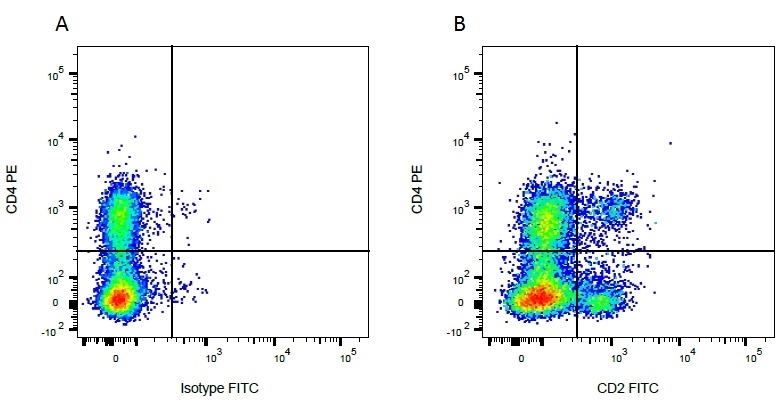
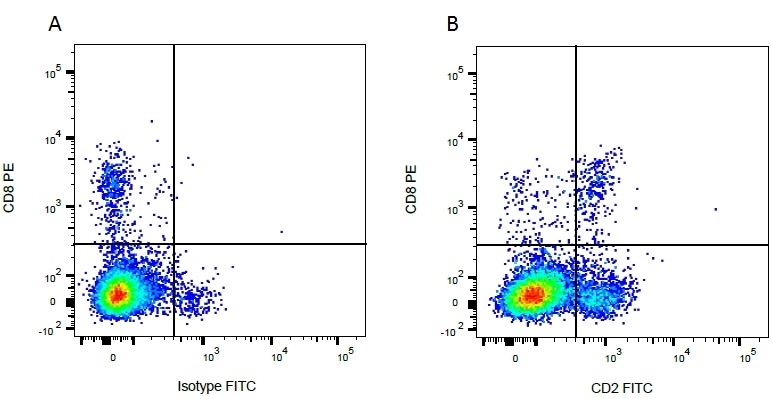
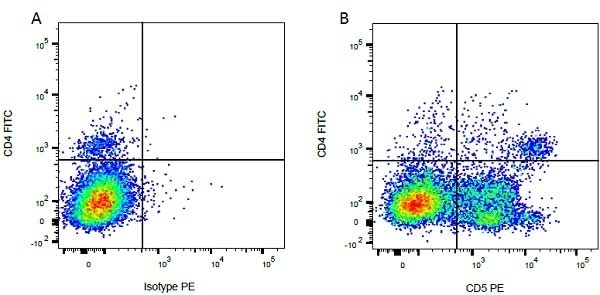
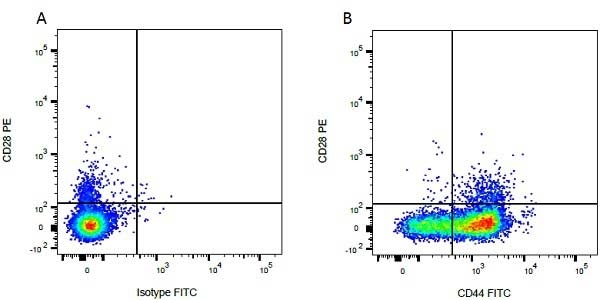
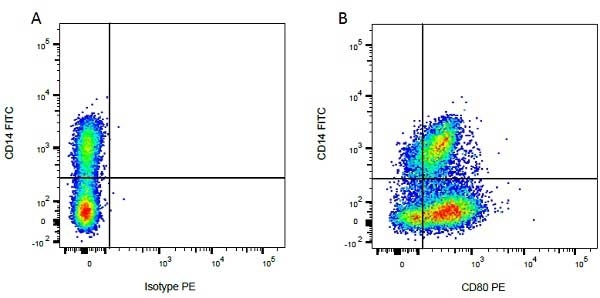
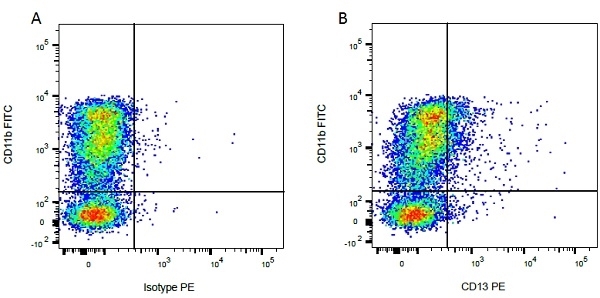
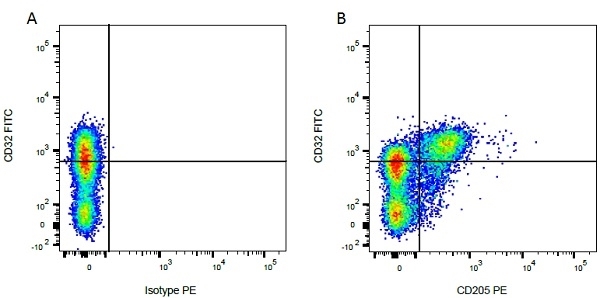
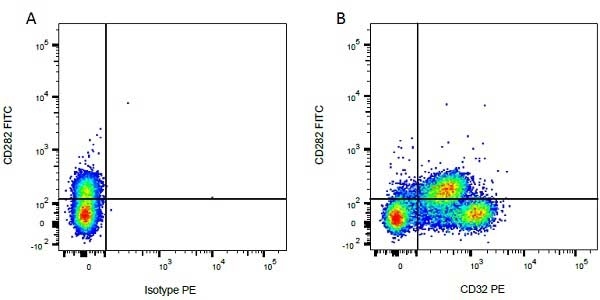
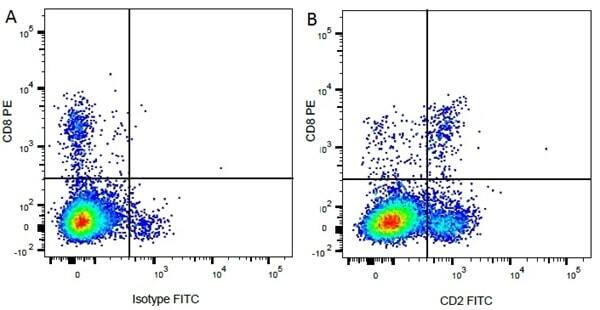
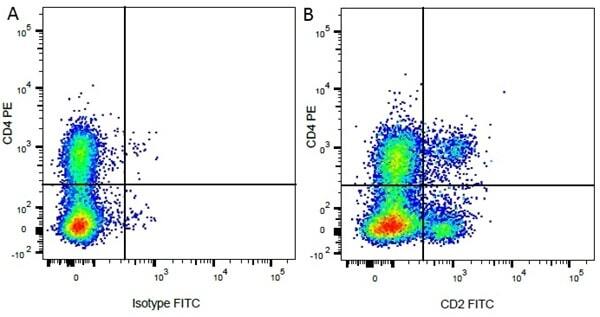
| Mouse IgG1 negative control is negative by flow cytometry on all human cells and cell lines tested. Further tests have also shown that this reagent is also suitable for use as a negative control with bovine ( This reagent recognizes a rat cell surface marker, and therefore cannot be used as a negative control in this species. |
- Target Species
- Negative Control
- Product Form
- Purified IgG conjugated to Pacific Blue® - liquid
- Preparation
- Purified IgG prepared by affinity chromatography on Protein A from tissue culture supernatant
- Buffer Solution
- Phosphate buffered saline
- Preservative Stabilisers
0.09% Sodium Azide 1% Bovine Serum Albumin - Approx. Protein Concentrations
- IgG concentration 0.05 mg/ml
- Max Ex/Em
-
Fluorophore Excitation Max (nm) Emission Max (nm) Pacific Blue® 410 455 - Regulatory
- For research purposes only
- Guarantee
- 12 months from date of despatch
- Acknowledgements
- The Pacific Blue® dye antibody conjugates in this product are sold under license from Molecular Probes, Inc. for research use only, except for use in combination with microarrays, and are covered by pending and issued patents.
Avoid repeated freezing and thawing as this may denature the antibody. Storage in frost-free freezers is not recommended. This product is photosensitive and should be protected from light.
| Application Name | Verified | Min Dilution | Max Dilution |
|---|---|---|---|
| Flow Cytometry | * |
*This antibody should be used at the same concentration as the test antibody.
- Flow Cytometry
- Use 10ul of the suggested working dilution to label 106 cells or 100ul whole blood.
How to Use the Spectraviewer
Watch the Tool Tutorial Video ▸- Start by selecting the application you are interested in, with the option to select an instrument from the drop down menu or create a customized instrument
- Select the fluorophores or fluorescent proteins you want to include in your panel to check compatibility
- Select the lasers and filters you wish to include
- Select combined or multi-laser view to visualize the spectra
References for Mouse IgG1 Negative Control antibody
-
Kupatt, C. et al. (2000) c7E3Fab reduces postischemic leukocyte-thrombocyte interaction mediated by fibrinogen. Implications for myocardial reperfusion injury.
Arterioscler Thromb Vasc Biol. 20 (10): 2226-32. -
Jacks, S. et al. (2007) Experimental infection of neonatal foals with Rhodococcus equi triggers adult-like gamma interferon induction.
Clin Vaccine Immunol.14:669-77 -
Pakandl, M. et al. (2008) Immune response to rabbit coccidiosis: a comparison between infections with Eimeria flavescens and E. intestinalis.
Folia Parasitol (Praha). 55:1-6. -
Dalli, J. et al. (2008) Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles.
Blood. 112 (6): 2512-9. -
Barratt-Due, A. et al. (2011) Ornithodoros moubata Complement Inhibitor Is an Equally Effective C5 Inhibitor in Pigs and Humans.
J Immunol. 187: 4913-9. -
Maślanka, T. et al. (2012) The presence of CD25 on bovine WC1+ gammadelta T cells is positively correlated with their production of IL-10 and TGF-beta, but not IFN-gamma.
Pol J Vet Sci. 15 (1): 11-20. -
Maiolini, A. et al. (2012) Toll-like receptors 4 and 9 are responsible for the maintenance of the inflammatory reaction in canine steroid-responsive meningitis-arteritis, a large animal model for neutrophilic meningitis.
J Neuroinflammation. 9: 226. -
Kapetanovic, R. et al. (2012) Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide.
J Immunol. 188: 3382-94.
View The Latest Product References
-
Kamble, N.M. et al. (2016) Interaction of a live attenuated Salmonella Gallinarum vaccine candidate with chicken bone marrow-derived dendritic cells.
Avian Pathol. 45 (2): 235-43. -
Iwaszko-Simonik, A. et al. (2015) Expression of surface platelet receptors (CD62P and CD41/61) in horses with recurrent airway obstruction (RAO).
Vet Immunol Immunopathol. 164 (1-2): 87-92. -
Brace, P.T. et al. (2017) Mycobacterium tuberculosis subverts negative regulatory pathways in human macrophages to drive immunopathology.
PLoS Pathog. 13 (6): e1006367. -
Topoluk, N. et al. (2017) Amniotic Mesenchymal Stromal Cells Exhibit Preferential Osteogenic and Chondrogenic Differentiation and Enhanced Matrix Production Compared With Adipose Mesenchymal Stromal Cells.
Am J Sports Med. 45 (11): 2637-46. -
Arzi, B. et al. (2017) Therapeutic Efficacy of Fresh, Allogeneic Mesenchymal Stem Cells for Severe Refractory Feline Chronic Gingivostomatitis.
Stem Cells Transl Med. 6 (8): 1710-22. -
Taechangam, N. et al. (2021) Feline adipose-derived mesenchymal stem cells induce effector phenotype and enhance cytolytic function of CD8+ T cells.
Stem Cell Res Ther. 12 (1): 495. -
do Prado Duzanski, A.et al. (2022) Cell-mediated immunity and expression of MHC class I and class II molecules in dogs naturally infected by canine transmissible venereal tumor: Is there complete spontaneous regression outside the experimental CTVT?
Research in Veterinary Science. 145: 193-204. -
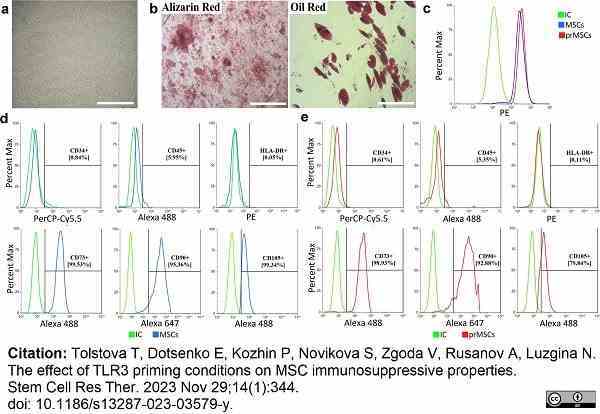
Tolstova, T. et al. (2023) The effect of TLR3 priming conditions on MSC immunosuppressive properties.
Stem Cell Res Ther. 14 (1): 344. -
Geng, Y. et al. (2018) Dietary vitamin D(3) supplementation protects laying hens against lipopolysaccharide-induced immunological stress.
Nutr Metab (Lond). 15: 58. -
Dan-Jumbo, S.O. et al. (2024) Derivation and long-term maintenance of porcine skeletal muscle progenitor cells.
Sci Rep. 14 (1): 9370. -
Maciag, S. et al. (2022) Effects of freezing storage on the stability of maternal cellular and humoral immune components in porcine colostrum.
Vet Immunol Immunopathol. 254: 110520. -
Forner, R. et al. (2021) Distribution difference of colostrum-derived B and T cells subsets in gilts and sows.
PLoS One. 16 (5): e0249366.
- RRID
- AB_567338
MCA928PB
If you cannot find the batch/lot you are looking for please contact our technical support team for assistance.
Please Note: All Products are "FOR RESEARCH PURPOSES ONLY"
View all Negative Control ProductsAlways be the first to know.
When we launch new products and resources to help you achieve more in the lab.
Yes, sign me up