Mini Review: Porcine Dendritic Cells
Introduction
Dendritic cells (DCs), originally identified by Steinman and Cohn in mice, are derived from common myeloid progenitors (CMPs) in the bone marrow. They are classified as the quintessential antigen-presenting cells (APCs), functioning as immune system sentinels, and are responsible for both the initiation of immune responses as well as the induction of immune self-tolerance (Steinman and Cohn 1973, Steinman and Witmer 1978). DCs express multiple pattern recognition receptors (PRRs), that identify the pathogen-associated molecular patterns (PAMPs) of viruses, bacteria, and protozoa at the site of infection, such as the skin and mucosa. After capturing and processing proteins, they migrate to secondary lymphoid tissues to present pathogen-derived antigens to lymphocytes (Banchereau and Steinman 1998).
At the time of their discovery, their role as APCs was recognized; however, their placement within the hematopoietic cell lineage is not fully settled yet. To date, several subsets have been defined (Guilliams et al. 2014).
In this mini review, we provide an overview of DCs and their subtypes, as well as their characterization in porcine blood, skin, tonsils, and lungs. In addition, we briefly describe the generation of monocyte-derived DCs (moDCs).
Dendritic Cell Subtypes
DCs are categorized into three types. Conventional DCs (cDCs) are the most potent APC, consisting of two subtypes. The cDC1 subset is efficient at antigen cross-presentation — acquiring exogenous antigens, presenting these via major histocompatibility complex (MHC) I molecules to CD8+ T cells, and initiating a type I immune response. The cDC2 subtype is more specialized, activating a CD4+ T cell response via MHC II antigen presentation. While these are the predominantly accepted roles for cDC1 and cDC2 subsets, some cross-functionality between the subsets remains to be elucidated (for a review see: Murphy and Murphy 2022).
The second type of DCs, plasmacytoid DC (pDCs), are mainly programmed to respond to viral infections via Toll‐like receptor 7 (TLR7) and TLR9, leading to rapid and copious secretion of type I and type III interferons and certain cytokines and chemokines (Collin and Bigley 2018, Reizis 2019).
Monocytes can be induced to exhibit some cDC properties, but do not align with the definition of cDC1s or cDC2s. Apart from in vitro experiments, moDCs have been found in human and mouse mucosal tissues associated with inflammatory conditions (Segura and Amigorena 2013, Tamoutounour et al. 2013, McGovern et al. 2014).
Defining Dendritic Cells in Pigs
Initial research in pigs focused on ‘natural interferon-producing cells’ (NIPCs), a precursor to DCs, ultimately leading to DC research. NIPCs were first identified by their ability to produce interferon-α (IFNα) after incubation with cells infected by a coronavirus, the transmissible gastroenteritis virus (TGEV) (Charley and Lavenant 1990).
Further studies on the TGEV model defined the origin and migration of NIPCs. Detection of these cells was initially limited to measuring the secretion of IFNα (Splíchal et al.1997). However, phenotypic characterization became possible using antibodies to SWC3 (Riffault et al. 2001). While early two-color flow cytometry had shown that SWC3 was highly expressed on myeloid cells (Saalmüller and Reddehase 1988), it was follow-on work that demonstrated expression on thymic DCs (Salmon et al. 2000), monocyte-derived and bone marrow-derived DCs (Carrasco et al. 2001), lamina propria DCs (Haverson et al. 2000), and dermal DCs (Bautista et al. 2002). Investigations by Summerfield defined the CD4−CD14− blood DC subset and the CD4++CD14− NIPCs by flow cytometry (Summerfield et al. 2003). SWC3 is a member of the signal-regulatory protein (SIRP) family and has been classified as CD172a (Alvarez et al. 2000).
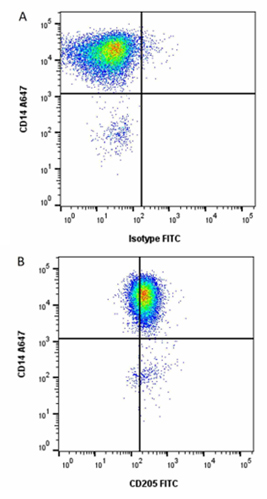
Fig. 1. Detecting pig blood DCs using Anti-Pig CD14 (MCA1218A647) and Anti-Pig CD205 (MCA6112F) in red cell lysed porcine blood.
Blood DCs
The key characterization of porcine blood DC subsets using multicolor flow cytometry identified CD172a+CD14−CD4− cells, similar to human cDC, making up 0.2–0.6% of peripheral blood mononuclear cells (PBMC) and CD172a+CD14−CD4++ cells, resembling human pDC, at 0.1–0.3% of PBMCs (Summerfield et al. 2003). 50–80% of CD4−CD14− cells expressed CD1 but this marker was not expressed on the CD4++CD14− cells. The CD4−CD14− subset also showed high MHC class II expression, whereas a lower expression was observed on the CD4++CD14− cells.
Porcine blood cDCs were summarized as MHC II+CD80/86+CD1+/−CD4−CD14−, upregulating MHC II and CD80/86 in response to culture with granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-3 (IL-3). Blood pDCs presented as a CD4+MHC IIlowCD80/86low CD1−CD8−/lowCD16−/lowCD45RA−/low phenotype, with IL-3 binding capacity enhancing their survival in vitro (Summerfield et al. 2003). As expected, following TGEV stimulation pDCs produced notable levels of IFNα. Both DC subsets exhibited robust endocytic activity immediately after isolation, diminishing upon in vitro cultivation (Summerfield et al. 2003).
This initial characterization of porcine blood DCs, drawing on similarities to human DCs, was extended years later by the same research group with a large panel of antibodies using multicolor flow cytometry, cell sorting, and RNA sequencing to define cDC1s, cDC2s, and pDCs in more detail (Auray et al. 2016):
- cDC1 as CD135+CD14-CD172alowCADM1+wCD11R1+ cells
- cDC2 as CD135+CD14-CD172a+CADM1+CD115+wCD11R1+CD1+ cells
- pDCs as CD4+CD135+CD172a+CD123+CD303+ cells
Other notable porcine CD marker expression characteristics showed the highest levels of CD205 (Figure 1) staining on the cDC1 subset, low surface expression of CD163 on the cDC2 subset, none of which were detected on cDC1s or pDCs (Auray et al. 2016). Additionally, Siglec-5 was expressed at low levels on pDCs (Escalona et al. 2014).
Skin DCs
Work on porcine skin DCs revealed the expected expression of CD1, CD172a, MHC II, and CD80/CD86, while CD11c, CD14, and CD16 were found to be variably expressed (Bautista et al. 2002). Langerhans cells (LCs) represent between 50-70% of DCs in porcine skin and are identified by the expression of Langerin (CD207) (Nfon et al. 2008). Extending this work by multicolor flow cytometry defined LCs as CD163−CD172a+CD16-CADM1+CD207highMHC II+ (Marquet et al. 2011). Marquet’s group also identified an additional three types of dermal DCs capable of migrating from the skin to local draining lymph nodes:
- Subtype 1: CD163−CD172a−
- Subtype 2: CD163highCD172a+
- Subtype 3: CD163lowCD172a+
Subtype 1 has been likened to mouse (CD172a−/CD103+) and human (CD172a−/BDCA3+) dermal DCs, able to cross-present antigens to CD8+ T cells. Subtype 2 also expressed CD206 and CD209 — inflammatory markers for moDCs and macrophages in humans and mice, indicating a possible monocytic origin for these DCs (Summerfield et al. 2015). Subtype 3 shares features with human CD1a+ dermal cDC2 and skews CD4+ T cells toward a Th17 profile (Marquet et al. 2014).
Tonsil DCs
Initially, two populations of DCs were defined in porcine tonsils, one mirroring cDCs (CD172a+CD11R1+CD1+/−CD80/86+/−) and the other pDCs (CD172a+CD4+CD1+/−CD80/86+/−) (Jamin et al. 2006). Later research on pig tonsils found five cell populations. Three of these were DC subsets: pDCs, a cDC1 subset with CD172a neg/low CADM1 high MHC II high, and CD172a high CD14−CD163−MHC II high CADM1 low cells representing cDC2s. The other two were considered to be monocyte-derived (CD172a high, CD163−CD14+MHC II high) and macrophage-like (CD172a highCD163+CD14−MHC II high) (Soldevila et al. 2018).
Porcine tonsil DCs were similarly characterized by another research group which extended the porcine DC flow immunophenotyping to CD205. Both CD205+ and CD205− DCs, irrespective of cDC1 or cDC2 subtype, were found in tonsils and other lymphoid tissues (Parra-Sanchez et al. 2018). This contrasts with blood where all DCs were CD205+ (Auray et al. 2016).
Lung DCs
The pig has been recognized as a valuable translational research model, e.g. for lung transplantation and influenza A infection (IAV) research. Using an IAV model in pigs, three distinct DC subsets were identified in the lung (Maisonnasse et al. 2016):
- cDC1: CD172a−XCR1+CadM1+CD11b-like−/MR-like−
- cDC2: CD163−CD172a+FcɛRiα+CD207+XCR1−
- Inflammatory moDC: CD163lowCD64intFcɛRiα−
The cDC1 cells showed better activation of CD8+ than CD4+ naive allogeneic T cells, leading to a Th1 response; but they did not induce antigen-specific proliferation of effector/memory CD8+ T cells (Maisonnasse et al. 2016). This characteristic led to the suggestion that porcine cDC1 cells behave more like mouse than human cDC1 cells. As expected, cDC2 cells activated CD8+ and CD4+ naive allogeneic T cells and induced a Th2 response.
However, relevant for translational research, they behaved more like their human counterparts in their close localization to tracheal and bronchial epithelia and expression of CD207 and FcERIα. In the context of IAV infection, human cDC2 cells are key to the induction of anti-IAV CD8+ T cell responses (Maisonnasse et al. 2016).
DCs Generated From Monocytes
DCs are only found at low levels in the circulation and other tissues making them difficult to study. One way to overcome this is to generate moDCs. Growing monocytes in a medium supplemented with recombinant GM-CSF and IL-4 gives rise to moDCs — MHC IIhighCD80/86+CD172a+CD1+ cells with a dendritic morphology and potent T cell stimulatory capacity (Carrasco et al. 2001, Paillot et al. 2001). In contrast to CD163− monocytes, CD163+ cells led to more mature moDCs, expressing higher levels of MHC II and CD80/86 and inducing more efficient lymphoproliferation (Chamorro et al. 2004).
To assess whether moDCs are comparable to DCs isolated from blood, i.e., pDCs and cDCs, functional studies were conducted. It was found that in pig blood isolated DCs were less able to stimulate naive T cell proliferation than moDC but induced identical proliferation of primed T cells. Blood-isolated DCs have lower endocytic activity than moDCs and a lower expression of CD80/86, in addition to lower basal cytokine protein concentrations.
Summary
In summary, the core function of DCs is to process antigens and present them to T cells to initiate immune responses. In addition to their basic immune defense function, they are also key to anti-tumor responses, vaccine development, and transplant allograft rejection. This mini review provides a starting point to guide marker selection for multicolor flow cytometry-based DC characterization (Table 1).
Table 1. DC populations and their key markers
DC Population |
DC Subtypes |
Key Markers |
|---|---|---|
|
Blood |
cDC1 |
CD135+CD14-CD172alowCADM1+wCD11R1+ |
|
cDC2 |
CD135+CD14-CD172a+CADM1+CD115+wCD11R1+CD1+ |
|
|
pDCs |
CD4+CD135+CD172a+CD123+CD303+ |
|
|
Skin |
Subtype 1 |
CD163−CD172a− |
|
Subtype 2 |
CD163highCD172a+ |
|
|
Subtype 3 |
CD163lowCD172a+ |
|
|
Tonsil |
cDC1 |
CD172aneg/lowCADM1highMHC IIhigh |
|
cDC2 |
CD172ahighCD14−CD163−MHC IIhighCADM1low |
|
|
pDCs |
CD172a+CD4+CD1+/−CD80/86+/− |
|
|
Lung DCs
|
cDC1 |
CD172a−XCR1+CadM1+CD11b-like−/MR-like− |
|
cDC2 |
CD163−CD172a+FcɛRiα+CD207+XCR1− |
|
|
inflammatory moDC |
CD163lowCD64intFcɛRiα− |
Porcine Antibodies
The porcine markers linked in this mini review are available from Bio-Rad in a variety of formats suitable for multicolor flow cytometry. Several markers are available conjugated to StarBright™ Dyes, designed to enable building larger and better flow panels for deeper immune cell analysis. Select from Bio-Rad’s porcine CD marker antibody range for full immune system phenotyping and complement it with cytokine and chemokine ELISA pairs for immune function analysis.
Bio-Rad’s veterinary species reactive antibodies are ideal for translational research studies, improving animal welfare, and advancing knowledge on One Health issues.
References
- Alvarez B et al. (2000). A porcine cell surface receptor identified by monoclonal antibodies to SWC3 is a member of the signal regulatory protein family and associates with protein-tyrosine phosphatase SHP-1. Tissue Antigens 55, 342–351.
- Auray G et al. (2016). Characterization and transcriptomic analysis of porcine blood conventional and plasmacytoid dendritic cells reveals striking species-specific differences. J Immunol 197, 4791–4806.
- Banchereau J and Steinman RM (1998). Dendritic cells and the control of immunity. Nature 392, 245–252.
- Bautista EM et al. (2002). Characterization and functional analysis of skin-derived dendritic cells from swine without a requirement for in vitro propagation. Vet Immunol Immunopathol. 88, 131–148.
- Carrasco CP et al. (2001). Porcine dendritic cells generated in vitro: morphological, phenotypic and functional properties. Immunology 104, 175–184.
- Chamorro S et al. (2004). In vitro differentiation of porcine blood CD163- and CD163+ monocytes into functional dendritic cells. Immunobiol 209, 57–65.
- Charley B and Lavenant L (1990). Characterization of blood mononuclear cells producing IFN alpha following induction by coronavirus-infected cells (porcine transmissible gastroenteritis virus). Res Immunol 141, 141–151.
- Collin M and Bigley V (2018). Human dendritic cell subsets: an update. Immunology 154, 3–20.
- Escalona Z et al. (2014). Molecular characterization and expression of porcine Siglec-5. Dev Comp Immunol 44, 206–216.
- Guilliams, M et al. (2014). Dendritic cells, monocytes and macrophages: a unified nomenclature based on ontogeny. Nat Rev Immunol 14, 571–578.
- Haverson K et al. (2000). Professional and non-professional antigen-presenting cells in the porcine small intestine. Immunology 101, 492–500.
- Jamin A et al. (2006). Characterization of conventional and plasmacytoid dendritic cells in swine secondary lymphoid organs and blood. Vet Immunol Immunopathol 114, 224–237.
- Marquet F et al. (2011). Characterization of dendritic cells subpopulations in skin and afferent lymph in the swine model. PloS One 6, e16320.
- Marquet F et al. (2014). Pig skin includes dendritic cell subsets transcriptomically related to human CD1a and CD14 dendritic cells presenting different migrating behaviors and T cell activation capacities. J Immunol 193, 5883–5893.
- Maisonnasse P et al. (2016). The respiratory DC/macrophage network at steady-state and upon influenza infection in the swine biomedical model. Mucosal Immunol 9, 835–849.
- McGovern N et al. (2014). Human dermal CD14⁺ cells are a transient population of monocyte-derived macrophages. Immunity 41, 465–477.
- Murphy L and Murphy KM (2022). Dendritic cells in cancer immunology. Cell Mol Immunol 19, 3–13.
- Nfon CK et al. (2008). Langerhans cells in porcine skin. Vet Immunol Immunopathol. 126, 236–247.
- Paillot R et al. (2001). Functional and phenotypic characterization of distinct porcine dendritic cells derived from peripheral blood monocytes. Immunol 102, 396–404.
- Parra-Sanchez H et al. (2018). Characterization and expression of DEC205 in the cDC1 and cDC2 subsets of porcine dendritic cells from spleen, tonsil, and submaxillary and mesenteric lymph nodes. Mol Immunol 96, 1–7.
- Reizis B (2019). Plasmacytoid dendritic cells: development, regulation, and function. Immunity 50, 37–50.
- Riffault S et al. (2001). Interferon-alpha-producing cells are localized in gut-associated lymphoid tissues in transmissible gastroenteritis virus (TGEV) infected piglets. Vet Res 32, 71–79.
- Salmon H et al. (2000). Dendritic cells enriched from swine thymus co-express CD1, CD2 and major histocompatibility complex class II and actively stimulate alloreactive T lymphocytes. Scand J Immunol 52, 164–172.
- Saalmüller A and Reddehase MJ (1988). Immune system of swine: dissection of mononuclear leucocyte subpopulations by means of two-colour cytofluorometric analysis. Res Vet Sci 45, 311–316.
- Segura E and Amigorena S (2013). Inflammatory dendritic cells in mice and humans. Trends Immunol 34, 440–445.
- Soldevila F et al. (2018). Characterization of the myeloid cell populations’ resident in the porcine palatine tonsil. Front Immunol 9, 1800.
- Splíchal I et al. (1997). In vivo study of interferon-alpha-secreting cells in pig foetal lymphohaematopoietic organs following in utero TGEV coronavirus injection. Res Immunol 148, 247–256
- Steinman RM and Cohn ZA (1973). Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 137, 1142–1162.
- Steinman RM and Witmer MD (1978). Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci USA 75, 5132–5136.
- Summerfield A et al. (2003). Porcine peripheral blood dendritic cells and natural interferon-producing cells. Immunology 110, 440–449.
- Summerfield A et al. (2015). The immunology of the porcine skin and its value as a model for human skin. Mol Immunol 66, 14–21.
- Tamoutounour, S et al. (2013). Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39, 925–938.